First principles study on stability and hydrogen adsorption properties of Mg/Ti interface†
Abstract
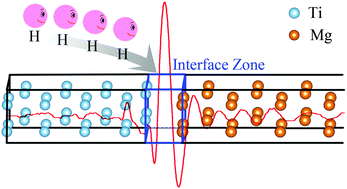
The hydrogenation and stability properties of the Mg/Ti interface are studied by first-principles calculations. The strain of lattice and movement of ions were imposed to search for a stable Mg/Ti interface. The anti-symmetrical configuration was found to be the most stable. The easiest transition pathway from anti-symmetrical to symmetrical configuration may be through the diagonal direction with no energy barrier. The hydrogen adsorption at distinguished positions in the Mg/Ti interface is investigated. The negative hydrogen adsorption energy reaches −0.991 eV at the top site in the interface, which will highly favor the thermodynamic stability of the Mg/Ti interface. The electronic structure is studied and it was found that the Ti acts as a hydrogen atom ‘capturer’ and strong interactions between H and its surrounding Ti and Mg atoms are expected. Thus, inserting Ti layers could create an interfacial zone where the adsorptions of hydrogen atoms may get stabilized and therefore improve the hydrogen storage properties of Mg.

 Please wait while we load your content...
Please wait while we load your content...