Promising gaseous and electrochemical hydrogen storage properties of porous Mg–Pd films under mild conditions
Abstract
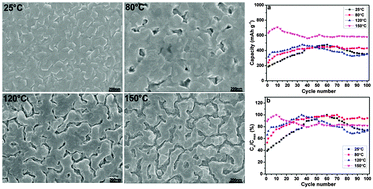
In this paper, the gaseous and electrochemical hydrogen storage properties of 200 nm Mg–Pd thin films with different morphologies have been investigated. The results show that Mg–Pd films become porous with the increase of substrate temperature. Porous Mg–Pd films exhibit superior gaseous and electrochemical hydrogen storage behaviors under mild conditions, including rapid hydrogen sorption kinetics, a large hydrogen storage amount, high electrochemical discharge capacity, and a fast hydrogen diffusion rate. The excellent behaviors of porous Mg–Pd films might be ascribed to the significantly shortened hydrogen diffusion paths and the large contact areas between the hydrogen gas and the solid Mg phases, which are elucidative for the development and applications of thick Mg–Pd films.

 Please wait while we load your content...
Please wait while we load your content...