Reply to the ‘Comment on “Charge Transfer to Solvent Dynamics in Iodide Aqueous Solution Studied at Ionization Threshold”’ by A. Lübcke and H.-H. Ritze, Phys. Chem. Chem. Phys., 2015, 17, DOI: 10.1039/C5CP00346F
Abstract
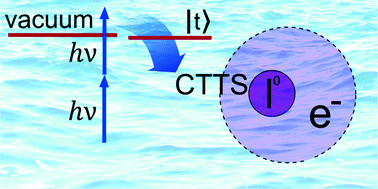
The role of experimental conditions in the study of the early-time charge transfer to solvent dynamics in iodide aqueous solution is revised. Under the short (∼50 fs) laser pulse regime of the current experiment, the presence of the pump–probe cross-correlation signal in the transient photoelectron spectra can be ruled out due to the much larger time scale of the electron-transfer dynamics. The ratio of the ionization yields from different initial states of iodide and water is argued to be dependent on the electron kinetic energy, and to be influenced by the presence of a bound resonance state above the vacuum threshold. Re-evaluation of our experimental data reassures the presence of an intermediate state in the charge-transfer process, initiated by electronic excitation into the continuum spectrum.

 Please wait while we load your content...
Please wait while we load your content...