Cation-assisted interactions between N-heterocycles and CO2†
Abstract
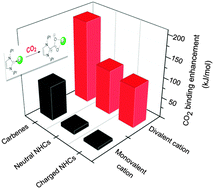
Cation-assisted interactions between N-containing heterocycles (NHCs) and CO2 have been systematically studied by using density functional theory (DFT). For neutral and anionic (non-carbenoid) NHCs, the effects of monovalent cations (i.e., alkali metal ions) are moderate to small (the NHC–CO2 binding energy change, ΔBE usually < 25 kJ mol−1). However, for NHC carbenes, due to their strong basicity, the effects are strong (ΔBE > 60 kJ mol−1) and the monovalent cations play a critical role in the single carboxylation of dicarbenes with CO2. In comparison, divalent alkali earth metal cations, due to both their smaller sizes and higher formal charges, exhibit a much stronger influence (ΔBE > 100 kJ mol−1). Divalent cations should be incorporated into next generation CO2 capture reagents. Other aspects including the reaction potential energy surface (PES), orbital-based analyses of interactions, substitution effects, and the reactivity descriptors (cation size, reacting N lone pair orbital energy, etc.) have been discussed in detail as well.

 Please wait while we load your content...
Please wait while we load your content...