Cationic gold clusters ligated with differently substituted phosphines: effect of substitution on ligand reactivity and binding
Abstract
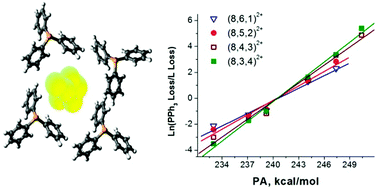
We present a systematic study of the effect of the number of methyl (Me) and cyclohexyl (Cy) functional groups in monodentate phosphine ligands on the solution-phase synthesis of ligated sub-nanometer gold clusters and their gas-phase fragmentation pathways. Small mixed ligand cationic gold clusters were synthesized using reactions between pre-formed triphenylphosphine ligated (PPh3) gold clusters and monodentate Me- and Cy-substituted phosphine ligands in solution and characterized using electrospray ionization mass spectrometry (ESI-MS) and collision-induced dissociation (CID) experiments. Under the same experimental conditions, larger gold–PPh3 clusters undergo efficient exchange of unsubstituted PPh3 ligands for singly Me- and Cy-substituted PPh2Me and PPh2Cy ligands. The efficiency of reaction decreases with an increasing number of Me or Cy groups in the substituted phosphine ligands. CID experiments performed for a series of mixed-ligand gold clusters indicate that loss of a neutral Me-substituted ligand is preferred over loss of a neutral PPh3 ligand while the opposite trend is observed for Cy-substituted ligands. The branching ratio of the competing ligand loss channels is strongly correlated with the electron donating ability of the phosphorous lone pair as determined by the relative proton affinity of the ligand. The results indicate that the relative ligand binding energies increase in the order PMe3 < PPhMe2 < PPh2Me < PPh3 < PPh2Cy < PPhCy2 < PCy3. Furthermore, the difference in relative ligand binding energies increases with the number of substituted PPh3−mMem or PPh3−mCym ligands (L) on each cluster. This study provides the first experimental determination of the relative binding energies of ligated gold clusters containing differently substituted monophosphine ligands, which are important to controlling their synthesis and reactivity in solution. The results also indicate that ligand substitution is an important parameter that must be considered in theoretical modeling of these complex systems.

 Please wait while we load your content...
Please wait while we load your content...