Spectral dependence of nonlinear optical properties of symmetrical octatetraynes with p-substituted phenyl end-groups†
Abstract
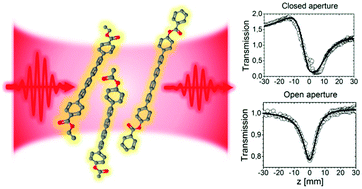
Organic compounds containing conjugated carbon chains have been extensively investigated due to their interesting properties including nonlinear optical response. Polyynes are a group of compounds where the conjugation can be modified by appropriate selection of end-groups, enabling “control” and improvement of nonlinear effects. In this work, we investigate three newly synthesized aryl end-capped octatetraynes, exhibiting strong nonlinear absorption and nonlinear refraction properties, which can be attributed to the presence of aryl end-groups with electron-withdrawing functional groups suitable for further extending the conjugation. Nonlinear optical measurements were performed using a f-scan (modified Z-scan) technique with a femtosecond laser system at various wavelengths in the visible and near-infrared range (540–1600 nm), revealing strong negative third-order nonlinear refraction (3NR) and two-photon absorption (2PA) below 600 nm and a noticeable three-photon absorption (3PA) at longer wavelengths. The results of spectroscopic characterization and the crystallographic data for the investigated compounds are also presented.

 Please wait while we load your content...
Please wait while we load your content...