Intriguing emission properties of triphenylamine–carborane systems†
Abstract
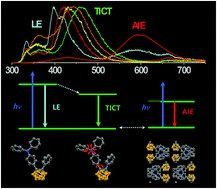
Electron donor–acceptor (D–A) systems with a triphenylamino moiety (D) and ortho-carborane (A) show three kinds of intriguing emissions that can be attributed to the local excited state, the intramolecular charge-transfer state, and the aggregation-induced emission state. The emission behaviors depend on which positions of the carborane are substituted.

 Please wait while we load your content...
Please wait while we load your content...