Bonding of heme FeIII with dioxygen: observation and characterization of an incipient bond
Abstract
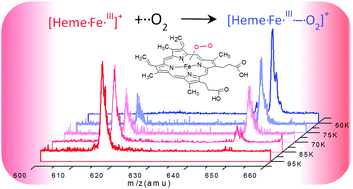
While ferrous heme (FeII) within hemoproteins binds dioxygen efficiently, it has not yet been possible to observe the analog complex with ferric heme (FeIII). We present the first observation and characterization of the latter complex in a cooled ion trap. The bond formation enthalpy of ferric heme–O2 has been derived from the Van't Hoff equation by means of temperature dependent measurements. The binding energy of the [heme FeIII–O2]+ ionic complex is rather strong as compared to that of [heme FeIII–N2]+, showing the formation of an incipient Fe–O bond, which is confirmed by the electronic absorption spectra of the two complexes. This first observation of the [heme FeIII–O2]+ complex lays the basis for the precise description of its electronic states.
- This article is part of the themed collection: Optical spectroscopy coupled with mass spectrometry methods

 Please wait while we load your content...
Please wait while we load your content...