A charge density study of π-delocalization and intermolecular interactions†
Abstract
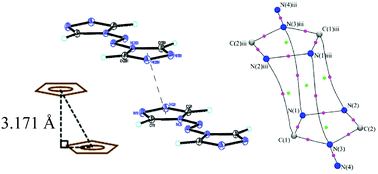
The compound trans-4,4′-azo-1,2,4-triazole (atrz) is a planar molecule with two planar triazole rings bridged by an azo group. The molecule is a good donor ligand and has an interesting π-delocalized character. In addition, intermolecular interactions in the crystalline state through π–π stacking are found between triazole rings with a very short inter-planar distance of 3.17 Å. The electron density distribution is obtained from both high resolution X-ray diffraction data at 100 K and density functional theory (DFT) calculations using the ωB97X-D functional. Bond characterization is performed in terms of the charge density distribution and the associated topological properties. The Laplacian distribution around each atom reveals the shape of the valence-shell charge concentration and demonstrates a sp2 hybrid orbital shape for each atom in the molecule. The π-delocalization of the planar molecule is further illustrated by the Fermi-hole distribution. The weak intermolecular π–π interactions and hydrogen bonds are further illustrated by the Hirshfeld surface. The energies of weak intermolecular π–π interactions and hydrogen bonds have been calculated using ωB97X-D/6-311++G(3df,2p) at experimental geometry.

 Please wait while we load your content...
Please wait while we load your content...