Assessing thermochemical properties of materials through ab initio quantum-mechanical methods: the case of α-Al2O3
Abstract
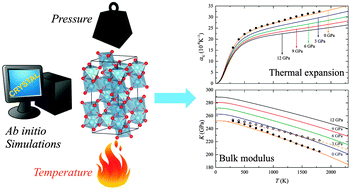
The thermochemical behavior of α-Al2O3 corundum in the whole temperature range 0–2317 K (melting point) and under pressures up to 12 GPa is predicted by applying ab initio methods based on the density functional theory (DFT), the use of a local basis set and periodic-boundary conditions. Thermodynamic properties are treated both within and beyond the harmonic approximation to the lattice potential. In particular, a recent implementation of the quasi-harmonic approximation, in the Crystal program, is here shown to provide a reliable description of the thermal expansion coefficient, entropy, constant-volume and constant-pressure specific heats, and temperature dependence of the bulk modulus, nearly up to the corundum melting temperature. This is a remarkable outcome suggesting α-Al2O3 to be an almost perfect quasi-harmonic crystal. The effect of using different computational parameters and DFT functionals belonging to different levels of approximations on the accuracy of the thermal properties is tested, providing a reference for further studies involving alumina polymorphs and, more generally, quasi-ionic minerals.

 Please wait while we load your content...
Please wait while we load your content...