Modulating the magnetic behavior of Fe(ii)–MOF-74 by the high electron affinity of the guest molecule†
Abstract
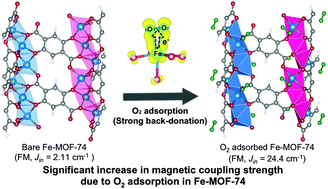
As a new class of magnetic materials, metal–organic framework (MOF) has received a significant attention due to their functionality and porosity that can provide diverse magnetic phenomena by utilizing host–guest chemistry. For Fe–MOF-74, we here find using density functional calculations that the O2 and C2H4 adsorptions result in the ferromagnetic (FM) and antiferromagnetic (AFM) orderings along the 1D chain of an hexagonal MOF framework, respectively, while their adsorption energies, pi-complexation, and geometrical changes are all similar upon binding. We reveal that this different magnetism behavior is attributed to the different electronic effects, where the adsorbed O2 greatly withdraws a minor spin electron from the Fe centers. The latter significant back donation opens a new channel for superexchange interactions that can enhance the FM coupling between Fe centers, where the strength of calculated intrachain FM coupling constrant (Jin) in O2 adsorbed Fe–MOF-74 is more than 10 times enhanced compared to bare Fe-MOF-74. This prediction suggests a possibility for the conceptual usage of Fe–MOF-74 as a gas sensor based on its magnetic changes caused by the adsorbed gases. Furthermore, the suggested mechanism might be used to control the magnetic properties of MOFs using the guest molecules, although concrete strategies to enhance such magnetic interactions to be used in practical applications would require further significant investigation.

 Please wait while we load your content...
Please wait while we load your content...