Ring-fused porphyrins: extension of π-conjugation significantly affects the aromaticity and optical properties of the porphyrin π-systems and the Lewis acidity of the central metal ions†
Abstract
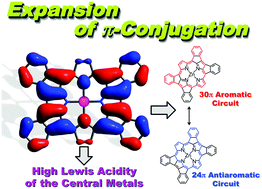
Here, we report the effects of ring fusion, which causes expansion of the π-conjugation circuits of the porphyrin derivatives to the fused meso-aryl groups, on the aromaticity and the magnetic properties of porphyrin derivatives. These studies revealed the facts that the ring fusion with five-membered rings causes not only the remarkable red shifts of the absorption bands and narrowed HOMO–LUMO gaps, but also the contribution of anti-aromatic resonance forms to the magnetic properties as observed in the 1H NMR spectra. The optical absorption and magnetic circular dichroism (MCD) spectroscopies indicate that the increase in the number of the fused rings causes stabilization of the LUMO level of the porphyrin derivatives and as a result induces the loosening of the LUMO degeneracy that is generally observed for porphyrins. The electronic structure of a quadruply fused porphyrin derivative was experimentally clarified by the ESR studies on the 1e−-oxidized and 1e−-reduced species in THF. Furthermore, we revealed the substituent effects of the fused meso-aryl groups of quadruply fused porphyrins (QFPs) on the crystal structures, absorption spectra and redox potentials; the sensitiveness of the substituent effects shows that the π-conjugation circuits extended to the fused meso-aryl groups. Additionally, the elongation of the bond lengths between the pyrrolic nitrogen and the central metal ions in QFP–metal complexes causes a remarkable increase of the Lewis acidity of the central metal ions.

 Please wait while we load your content...
Please wait while we load your content...