Colloidal properties and behaviors of 3 nm primary particles of detonation nanodiamonds in aqueous media†
Abstract
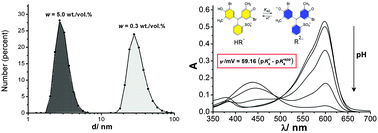
This study was aimed to reveal the principal colloidal properties of the aqueous dispersion of extremely small primary single-crystalline diamond particles in water. Together with the non-diamond layer, the size of the colloidal species is 2.8 ± 0.6 nm as found via DLS of the initial 5.00 wt/vol% hydrosol. Anionic dyes are readily adsorbed on the colloidal species. This is in line with the positive zeta-potential. The critical coagulation concentrations of the 0.19 wt/vol% nanodiamond hydrosol were determined with a set of inorganic electrolytes and anionic surfactants. The data are in line with the Schulze–Hardy rule for “positive” sols. The fulfillment of the lyotropic (Hofmeister) series was also observed for single-charged anions. The abnormal influence of alkali gives evidence of the acidic nature of the positive charge of the nanodiamond species. Application of acid–base indicators allows estimating the value of the interfacial electrical potential of the nanodiamond particles. Upon dilution from 5.00% to 0.01%, the colloidal system under study exhibits unusual changes. The average size increases ca. ten-fold as determined by DLS. The TEM images support this observation. At the same time, the viscosity drops. This phenomenon was explained in terms of the so-called periodic colloidal structures (colloidal crystals) in concentrated solutions.

 Please wait while we load your content...
Please wait while we load your content...