Excited state lifetimes and energies of okenone and chlorobactene, exemplary keto and non-keto aryl carotenoids
Abstract
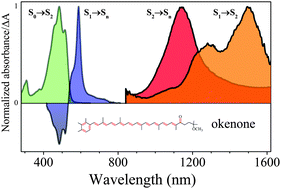
Photophysical properties of two typical aryl carotenoids, okenone and chlorobactene, were studied with application of femtosecond and microsecond time-resolved absorption spectroscopies. These carotenoids are structurally similar and differ only by keto-group and character of the aryl ring. The studies have concentrated on aspects of the photochemistry of these carotenoids as possibility of solvent polarity induced formation of intramolecular charge transfer state in okenone, which contains a keto-group directly attached to the carbon–carbon double bond conjugation, estimating the energy of the forbidden first excited singlet electronic state, S1 (21Ag−) and testing the photoprotective capabilities of okenone and chlorobactene in real biological systems. The energies of the S1 (21Ag−) state obtained for these carotenoids are 12 750 cm−1 for okenone and 13 450 cm−1 for chlorobactene and are not affected either by temperature or solvent polarity. The effect of cryogenic temperature on the excited states lifetimes and energies was also studied at 77 K in 2-methyltetrahydrofuran, which forms a transparent glass upon freezing. The ability to quench bacteriochlorophylls triplets was studied on model bacteriochlorophyll a–carotenoid mixtures with application of flash photolysis. The triplet state lifetime obtained from the anticipated kinetic modelling of the rise and decay of the pool of carotenoid triplets are 2.1 μs for okenone and 2.8 μs for chlorobactene.

 Please wait while we load your content...
Please wait while we load your content...