Enhancing electrochemical performance by control of transport properties in buffer layers – solid oxide fuel/electrolyser cells†
Abstract
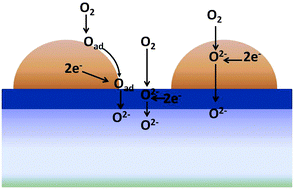
The current work demonstrates how tailoring the transport properties of thin ceria-based buffer layers in solid oxide fuel or electrolyser cells can provide the necessary phase stability against chemical interaction at the electrolyte/electrode interface, while also providing radical improvements in the electrochemical performance of the oxygen electrode. Half cells of Ce0.8R0.2O2−δ + 2 mol% Co buffer layers (where R = Gd, Pr) with Nd2NiO4+δ electrodes were fabricated by spin coating on dense YSZ electrolyte supports. Dramatic decreases in polarization resistance, Rp, of up to an order of magnitude, could be achieved in the order, Pr ≪ Gd < no buffer layer. The current article shows how this improvement can be related to increased levels of ambipolar conductivity in the mixed conducting buffer layer, which provides an additional parallel path for electrochemical reaction. This is an important breakthrough as it shows how electrode polarization resistance can be substantially improved, in otherwise identical electrochemical cells, solely by tailoring the transport properties of thin intermediate buffer layers.

 Please wait while we load your content...
Please wait while we load your content...