Experimental and theoretical study of the oxidation of ventilation air methane over Fe2O3 and CuO
Abstract
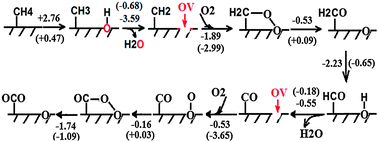
Coal mine ventilation air methane (VAM) is an important contributor to methane emissions from the energy sector. Although various technologies are under development, treatment of the VAM with an efficient and cost-effective approach has been an ongoing challenge due to massive flow rates of the ventilation air and low and variable methane concentrations. Recently a new concept based on the principle of chemical looping combustion (CLC) has been proposed for VAM abatement (Appl. Energy, 2014, 113, 1916), in which oxidation of low-concentration CH4 balanced by N2 with Fe2O3 or CuO as the oxygen carrier was studied. Here, we thoroughly examined the feasibility of CLC of VAM based on experimental study and theoretical calculations. Reduction of Fe2O3 and CuO and evolution of gas products during CH4 oxidation were investigated using TGA-MS under two reaction atmospheres: 1 vol% CH4 balanced by N2 and the simulated VAM containing 1 vol% CH4, 20 vol% O2, 0.4 vol% CO2 and balance N2. It was found that the CLC of VAM is fundamentally infeasible because the reduced phase of Fe2O3 and CuO cannot be formed for chemical looping when reacting with the simulated VAM containing abundant oxygen. Theoretical calculations revealed that Fe2O3 and CuO remain stable without the transition to the reduced phase as the generated oxygen vacancy on the surface of metal oxides during CH4 oxidation can recover quickly with O2 adsorption and dissociation. Calculations confirmed that both Fe2O3 and CuO play a role of surface catalyst in VAM oxidation. More importantly, it was found that the low-coordinated metal atoms and oxygen vacancies can stabilize CHx radicals to promote the dissociation of CH4, which is generally the rate-determining step for CH4 oxidation. Such findings are useful for new development and understanding of high-performance and low-cost metal oxide catalysts for CH4 oxidation.

 Please wait while we load your content...
Please wait while we load your content...