The role of the ionic liquid C6C1ImTFSI in the sol–gel synthesis of silica studied using in situ SAXS and Raman spectroscopy†
Abstract
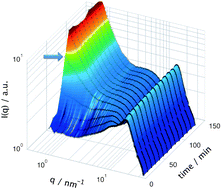
The sol–gel synthesis of a silica based ionogel using the ionic liquid 1-hexyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide (C6C1ImTFSI) as the solvent has been followed in situ by combined μ-focused X-ray scattering and μ-Raman spectroscopy. By covering the momentum transfer range 0.2 < q < 30 nm−1 we probe the evolution of the characteristic peaks of the ionic liquid, associated with the existence of polar and non-polar domains, as a function of reaction time. Our detailed analysis of the small angle X-ray scattered (SAXS) pattern reveals that the nano-structure of the ionic liquid is partially retained during the sol–gel synthesis, as indicated by the broader yet distinguishable SAXS signatures. We also observe that the signature associated with the non-polar and polar domains shift to higher and lower q-values, respectively. Interestingly, this behavior correlates with the evolution of the chemical composition of the sol as probed by Raman spectroscopy. More precisely, we observe that both the nano-structural changes and the production of polar molecules arrest at the point of gelation. This is rationalized by the tendency of the reagents and products of the sol–gel reaction to locate in different portions of the nano-structure of the ionic liquid.

 Please wait while we load your content...
Please wait while we load your content...