Ferromagnetic nanocrystallines containing copper as an efficient catalyst for photoinduced water oxidation†
Abstract
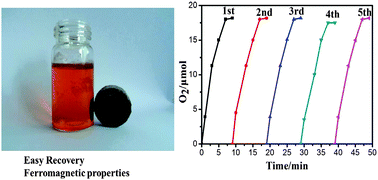
CuFe2O4 nanocrystallines with cubic jacobsite structure have been obtained by heat treatment of the coprecipitation product, which were synthesized by the reaction of Cu2+ ions and Fe3+ ions under alkaline conditions. Reported here is the first copper-based catalyst for photocatalytic water oxidation using [Ru(bpy)3]Cl2 as the photosensitizer and Na2S2O8 as the sacrificial electron acceptor, respectively. An apparent TOF value of 1.2 μmol s−1 m−2 and an oxygen yield of 72.8% were obtained with CuFe2O4. The apparent TOF value with CuFe2O4 (1.2 μmol s−1 m−2) is the highest value among all heterogeneous photocatalytic water oxidation systems. CuFe2O4 can be easily separated from reaction solution by magnetic separation while maintaining excellent water oxidation activity in the fourth and fifth runs. The surface conditions of CuFe2O4 are slightly absent after examination by X-ray photoelectron spectroscopy (XPS) before and after the photocatalytic reaction.

 Please wait while we load your content...
Please wait while we load your content...