Nanohydration of uracil: emergence of three-dimensional structures and proton-induced charge transfer†
Abstract
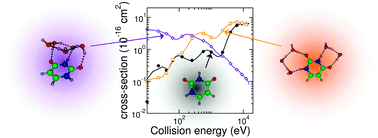
Stepwise hydration of uracil has been theoretically revisited using different methods ranging from classical force fields to quantum chemical approaches. Hydration initially begins within the uracil plane but proceeds at four molecules into three-dimensional configurations or even water clusters next to the nucleobase. The relative stability between the various structures is significantly affected by zero-point energy and finite temperature (entropy) effects and also gives rise to markedly different responses to an excitation by an impinging high-energy proton. In particular, charge transfer to the molecular complex is dramatically altered in collisions toward the coating cluster but barely modified for peripheral hydration patterns.

 Please wait while we load your content...
Please wait while we load your content...