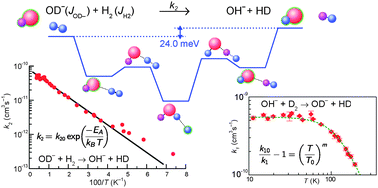
Using a cryogenic linear 22-pole rf ion trap, rate coefficients for H/D exchange reactions of OH− with D2 (1) and OD− with H2 (2) have been measured at temperatures between 11 K and 300 K with normal hydrogen. Below 60 K, we obtained k1 = 5.5 × 10−10 cm3 s−1 for the exoergic reaction (1). Upon increasing the temperature above 60 K, the data decrease with a power law, k1(T) ∼ T−2.7, reaching ≈1 × 10−10 cm3 s−1 at 200 K. This observation is tentatively explained with a decrease of the lifetime of the intermediate complex as well as with the assumption that scrambling of the three hydrogen atoms is restricted by the topology of the potential energy surface. The rate coefficient for the endoergic reaction (2) increases with temperature from 12 K up to 300 K, following the Arrhenius equation, k2 = 7.5 × 10−11 exp(−92 K/T) cm3 s−1 over two orders of magnitude. The fitted activation energy, EA-Exp = 7.9 meV, is in perfect accordance with the endothermicity of 24.0 meV, if one accounts for the thermal population of the rotational states of both reactants. The low mean activation energy in comparison with the enthalpy change in the reaction is mainly due to the rotational energy of 14.7 meV contributed by ortho-H2 (J = 1). Nonetheless, one should not ignore the reactivity of pure para-H2 because, according to our model, it already reaches 43% of that of ortho-H2 at 100 K.
This article is Open Access
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...