Synthesis and hydrolysis of gas-phase lanthanide and actinide oxide nitrate complexes: a correspondence to trivalent metal ion redox potentials and ionization energies†
Abstract
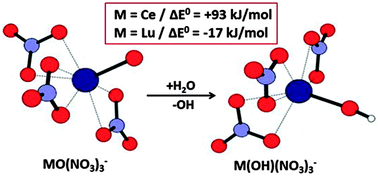
Several lanthanide and actinide tetranitrate ions, MIII(NO3)4−, were produced by electrospray ionization and subjected to collision induced dissociation in quadrupole ion trap mass spectrometers. The nature of the MO(NO3)3− products that result from NO2 elimination was evaluated by measuring the relative hydrolysis rates under thermalized conditions. Based on the experimental results it is inferred that the hydrolysis rates relate to the intrinsic stability of the MIV oxidation states, which correlate with both the solution IV/III reduction potentials and the fourth ionization energies. Density functional theory computations of the energetics of hydrolysis and atoms-in-molecules bonding analysis of representative oxide and hydroxide nitrates substantiate the interpretations. The results allow differentiation between those MO(NO3)3− that comprise an O2− ligand with oxidation to MIV and those that comprise a radical O− ligand with retention of the MIII oxidation state. In the particular cases of MO(NO3)3− for M = Pr, Nd and Tb it is proposed that the oxidation states are intermediate between M(III) and M(IV).

 Please wait while we load your content...
Please wait while we load your content...