A molecular dynamics study of CaCO3 nanoparticles in a hydrophobic solvent with a stearate co-surfactant†
Abstract
Stearates containing overbased detergent nanoparticles (NPs) are used as acid neutralising additives in automotive and marine engine oils. Molecular dynamics (MD) simulations of the self-assembly of calcium carbonate, calcium stearate as a co-surfactant and stabilising surfactants of such NPs in a model explicit molecular hydrophobic solvent have been carried out using a methodology described first by Bodnarchuk et al. [J. Phys. Chem. C, 2014, 118, 21092]. The cores and particles as a whole become more elongated with stearate, and the surfactant molecules are more spaced out in this geometry than in their stearate-free counterparts. The rod dimensions are found to be largely independent of the surfactant type for a given amount of CaCO3. The corresponding particles without stearate were more spherical, the precise shape depending to a greater extent on the chemical architecture of the surfactant molecule. The rod-shaped stearate containing nanoparticles penetrated a model water droplet to a greater depth than the corresponding near-spherical particle, which is possibly facilitated by the dissociation of nanoparticle surfactant molecules onto the surface of the water in this process. These simulations are the first to corroborate the nanoparticle–water penetration mechanism proposed previously by experimental groups investigating the NP acid neutralisation characteristics.
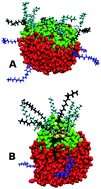

 Please wait while we load your content...
Please wait while we load your content...