Are intramolecular frustrated Lewis pairs also intramolecular catalysts? A theoretical study on H2 activation†
Abstract
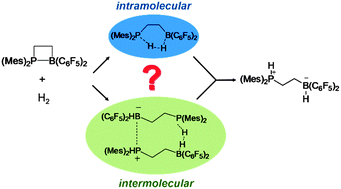
We investigate computationally a series of intramolecular frustrated Lewis pairs (FLPs), with the general formula Mes2PCHRCH2B(C6F5)2, that are known from the literature to either activate molecular hydrogen (FLPs with R = H (1) or Me (4)), or remain inert (FLPs with R = Ph (2) or SiMe3 (3)). The prototypical system Mes2PCH2CH2B(C6F5)2 (1) has been described in the literature (Grimme et al., Angew. Chem., Int. Ed., 2010; Rokob et al., J. Am. Chem. Soc., 2013) as an intramolecular reactant that triggers the reaction with H2 in a bimolecular concerted fashion. In the current study, we show that the concept of intramolecular H2 activation by linked FLPs is not able to explain the inertness of the derivative compounds 2 and 3 towards H2. To cope with this, we propose an alternative intermolecular mechanism for the investigated reaction, assuming stacking of two open-chain FLP conformers, and formation of a dimeric reactant with two Lewis acid–base domains, that can split up to two hydrogen molecules. Using quantum-chemical methods, we compute the reaction profiles describing these alternative mechanisms, and compare the derived predictions with earlier reported experimental results. We show that only the concept of intermolecular H2 activation could explain both the activity of the FLPs having small substituents in the bridging molecular region, and the inertness of the FLPs with a bulkier substitution, in a consistent way. Importantly, the intermolecular H2 activation driven by intramolecular FLPs indicates the key role of steric factors and noncovalent interactions for the design of metal-free systems that can efficiently split H2, and possibly serve as metal-free hydrogenation catalysts.

 Please wait while we load your content...
Please wait while we load your content...