Unconventional charge distribution in the planar wheel-type M©B6H6−/0/+ (M = Mn, Fe and Co): central M with negative charges and peripheral boron ring with positive charges
Abstract
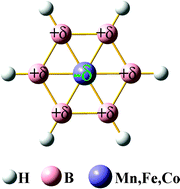
Planar wheel-type D6h M©B6H6−/0/+ (M = Mn, Fe and Co for anion, neutral and cation, respectively.) clusters with a planar hexacoordinate transition-metal at the center of the boron ring were designed and investigated by density functional theory. These planar clusters are chemically stable as a result of their large binding energy, vertical ionization potential, and vertical electron affinity. The detailed natural population and molecular orbital analyses suggest that not only does the M atom donate electrons to the boron ring for participation in the π-delocalized bonding, but also the boron ring donates electrons back to the M atom for the formation of the σ-delocalized bonding, which leads to a strong aromaticity and unconventional charge distribution, i.e., the M atom is negatively charged, while the boron ring is positively charged. This study may open a new area in coordination chemistry for planar hexacoordinate transition metals and we expect further experimental exploration of their synthesis and potential applications.

 Please wait while we load your content...
Please wait while we load your content...