Methyl-branched lipids promote the membrane adsorption of α-synuclein by enhancing shallow lipid-packing defects
Abstract
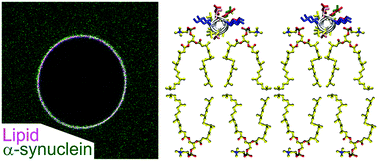
Alpha-synuclein (AS) is a synaptic protein that is directly involved in Parkinson's disease due to its tendency to form protein aggregates. Since AS aggregation can be dependent on the interactions between the protein and the cell plasma membrane, elucidating the membrane binding properties of AS is of crucial importance to establish the molecular basis of AS aggregation into toxic fibrils. Using a combination of in vitro reconstitution experiments based on Giant Unilamellar Vesicles (GUVs), confocal microscopy and all-atom molecular dynamics simulations, we have investigated the membrane binding properties of AS, with a focus on the relative contribution of hydrophobic versus electrostatic interactions. In contrast with previous observations, we did not observe any binding of AS to membranes containing the ganglioside GM1, even at relatively high GM1 content. AS, on the other hand, showed a stronger affinity for neutral flat membranes consisting of methyl-branched lipids. To rationalize these results, we used all-atom molecular dynamics simulations to investigate the influence of methyl-branched lipids on interfacial membrane properties. We found that methyl-branched lipids promote the membrane adsorption of AS by creating shallow lipid-packing defects to a larger extent than polyunsaturated and monounsaturated lipids. Our findings suggest that methyl-branched lipids may constitute a remarkably adhesive substrate for peripheral proteins that adsorb on membranes via hydrophobic insertions.
- This article is part of the themed collection: Chemical compartmentalisation by membranes: from biological mechanism to biomimetic applications

 Please wait while we load your content...
Please wait while we load your content...