On the relationship between the basicity of a surface and its ability to catalyze transesterification in liquid and gas phases: the case of MgO
Abstract
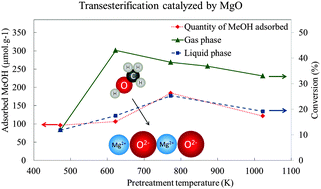
Gas or liquid phase transesterification reactions are used in the field of biomass valorization to transform some platform molecules into valuable products. Basic heterogeneous catalysts are often claimed for these applications but the role of basicity in the reaction mechanism depending on the operating conditions is still under debate. In order to compare the catalyst properties necessary to perform a transesterification reaction both in liquid and gas phases, ethyl acetate and methanol, which can be easily processed both in these two phases, were chosen as reactants. The catalyst studied is MgO, known for its basic properties and its ability to perform the reaction. By means of appropriate thermal treatments, different kinds of MgO surfaces, with different coverages of natural adsorbates (carbonates and hydroxyls groups), can be prepared and characterized by means of CO2 adsorption followed by IR spectroscopy and hept-1-ene isomerization model reaction. New results on the basicity of the natural MgO surface (covered by carbonate and hydroxyl groups) are first given and discussed. The catalytic behavior in the transesterification reaction is then determined as a function of the adsorbate coverage. It is shown that the transesterification activity in the liquid phase is directly correlated with the kinetic basicity of the surface in agreement with the mechanism already proposed in the literature. On the reverse, no direct correlation with the basicity of the surface was established with the transesterification activity in the gas phase. A very high activity, in the gas phase, was observed and discussed for the natural surface pre-treated at 623 K. Preliminary DFT modeling of ester adsorption and methanol adsorption capacity determination were performed to investigate plausible reaction routes.

 Please wait while we load your content...
Please wait while we load your content...