A comprehensive study on micellization of dissymmetric pyrrolidinium headgroup-based gemini surfactants†
Abstract
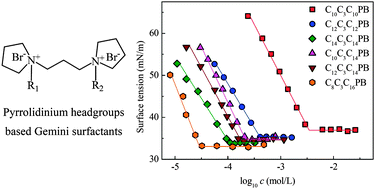
Three groups of pyrrolidinium headgroup-based gemini surfactants of 1,1′-(propane-1,3-diyl)bis(1-alkyl pyrrolidinium) bromide, in categories of symmetric CmC3CmPB (m = 10, 12, 14), dissymmetric CmC3C14PB (m = 10, 12, 14) and CmC3CnPB (m = 8, 10, 12, m + n = 24) surfactants, are studied using equilibrium surface tension, conductivity, fluorescence, and NMR techniques. The importance of the dissymmetry on the micellization has been revealed in detail. The increase in the hydrophobic chain length m for CmC3CmPB and CmC3C14PB or in the dissymmetry (n/m) for CmC3CnPB can strengthen the aggregation ability and surface activity of the surfactants significantly, i.e., a lower critical micelle concentration (cmc) and a lower surface tension at cmc (γcmc). However, the aggregation number at cmc (N*) obeys the opposite variation tendency and it becomes smaller upon increasing m or n/m, due to the formation of premicelles. Thermodynamic results reveal that the contribution of enthalpy (ΔH0m) to the Gibbs free energy (ΔG0m) is strengthened by increasing m or n/m during the spontaneous micellization process. Moreover, 1H NMR results confirm the microenvironment change of the surfactants from polar water to micelles during the micellization, and 2D Noesy NMR spectra suggest that the methylene groups in the ring should adopt a conformation toward the nonpolar micellar core rather than in the polar water.

 Please wait while we load your content...
Please wait while we load your content...