The role of the shape resonance state in low energy electron induced single strand break in 2′-deoxycytidine-5′-monophosphate†
Abstract
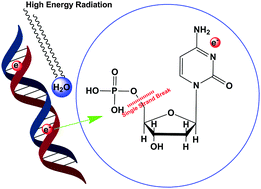
Low energy electron (LEE) induced single strand break (SSB) has been studied for 2′-deoxycytidine-5′-monophosphate (5′-dCMPH) molecules in the gas phase by means of ab initio electronic structure methods and local complex potential based time-dependent wavepacket quantum mechanical calculations. We have found that the LEE attachment to this cytidine nucleotide results in the formation of a transient metastable anion. The results obtained here show that the electron attachment takes place at the cytosine nucleobase center and within 18–20 fs, the LEE transfers to the σ* orbital of the sugar-phosphate 5′ C–O bond. The characteristic electron attachment cross section spectrum is found at ∼1 eV, which is in good agreement with the available experimental observations. Quantum mechanical tunneling of the 5′ C–O bound vibrational energy levels may contribute to SSB only above 1.5 eV energy regimes.


 Please wait while we load your content...
Please wait while we load your content...