Estimating successive pKa values of polyprotic acids from ab initio molecular dynamics using metadynamics: the dissociation of phthalic acid and its isomers†
Abstract
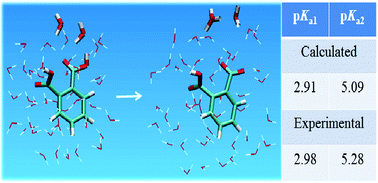
Estimation of the dissociation constant, or pKa, of weak acids continues to be a central goal in theoretical chemistry. Here we show that ab initio Car–Parrinello molecular dynamics simulations in conjunction with metadynamics calculations of the free energy profile of the dissociation reaction can provide reasonable estimates of the successive pKa values of polyprotic acids. We use the distance-dependent coordination number of the protons bound to the hydroxyl oxygen of the carboxylic group as the collective variable to explore the free energy profile of the dissociation process. Water molecules, sufficient to complete three hydration shells surrounding the acid molecule, were included explicitly in the computation procedure. Two distinct minima corresponding to the dissociated and un-dissociated states of the acid are observed and the difference in their free energy values provides the estimate for pKa, the acid dissociation constant. We show that the method predicts the pKa value of benzoic acid in good agreement with experiment and then show using phthalic acid (benzene dicarboxylic acid) as a test system that both the first and second pKa values as well, as the subtle difference in their values for different isomers can be predicted in reasonable agreement with experimental data.

 Please wait while we load your content...
Please wait while we load your content...