The self assembly of proteins; probing patchy protein interactions†
Abstract
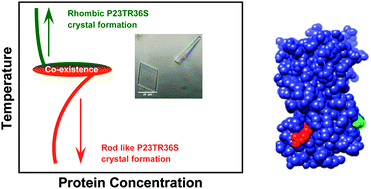
The ability to control the self-assembly of biological molecules to form defined structures, with a high degree of predictability is a central aim for soft matter science and synthetic biology. Several examples of this are known for synthetic systems, such as anisotropic colloids. However, for biomacromolecules, such as proteins, success has been more limited, since aeolotopic (or anisotropic) interactions between protein molecules are not easily predicted. We have created three double mutants of human γD-crystallin for which the phase diagrams for singly mutated proteins can be used to predict the behavior of the double mutants. These proteins provide a robust mechanism to examine the kinetic and thermodynamic properties of proteins in which competing interactions exist due to the anisotropic or patchy nature of the protein surface.

 Please wait while we load your content...
Please wait while we load your content...