Temperature-induced amorphisation of hexagonal ice
Abstract
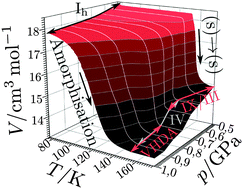
We systematically studied the competition between polymorphic transformations and amorphisation of hexagonal ice on isobaric heating from 77 K to 155–170 K at pressures between 0.50 and 1.00 GPa. This competition is analysed here systematically by in situ dilatometry and ex situ X-ray diffraction and calorimetry. Volume vs. temperature curves were analysed using a novel fitting approach in order to understand the underlying mechanism. Hexagonal ice undergoes solid-state-transformation to ice IX/III at 0.50 and 0.60 GPa and to a mixture of ices IX/III and IV at 0.70 and 0.80 GPa. Possibly a tiny fraction of amorphous intermediate is transiently formed in this pressure range. At 0.85 GPa the amorphisation process becomes competitive, and leads to very high-density amorphous ice (VHDA) as by-product. At 0.90 and 0.95 GPa VHDA is the main product and at 1.00 GPa only VHDA is found. This represents the first observation of temperature-induced amorphisation (TIA) for hexagonal ice using diffraction methods. Our analysis suggests TIA to be a first-order phase transition which, by contrast to pressure-induced amorphisation (PIA), does not involve a precursor process. We suggest interpreting TIA as thermodynamic melting of ice followed by immediate vitrification rather than as mechanical collapse of hexagonal ice. The activation energies for amorphisation and polymorphic transformation are equal at ∼0.75 GPa. At 1.00 GPa the activation energy for amorphisation of hexagonal ice is lower by about 6 kJ mol−1 than the activation energy for polymorphic transitions.

 Please wait while we load your content...
Please wait while we load your content...