Poly(3-hydroxybutyrate-co-4-hydroxybutyrate) based nanocomposites: influence of the microstructure on the barrier properties
Abstract
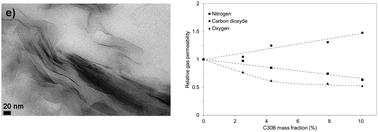
Poly(3-hydroxybutyrate-co-4-hydroxybutyrate) (P3HB-co-4HB) films containing various contents of organo-modified montmorillonite C30B nanoclays were prepared by melt intercalation. Wide angle X-ray diffraction measurements and transmission electron microscopy observations evidenced aggregated and intercalated structures with individual nanoclay platelets in the nanocomposites and an orientation of nanoclays. Differential scanning calorimetry measurements showed that the nanoclay did not influence the crystalline structure of the matrix because it is mainly located in the polymer amorphous phase. The influence of the filler on the barrier properties of the film was evaluated by water diffusion, gas permeation (CO2, N2, O2) and liquid water sorption measurements. A decrease of the N2 permeability was measured due to the tortuosity effect of the filler associated with a decrease of the solubility within the matrix. The influence of the filler was more marked for O2 due to the larger decrease of O2 solubility. In contrast, the CO2 permeability increased whatever the filler content because of a facilitated transport mechanism due induced by the presence of quaternary ammonium cations on the C30B surface. The decrease of the water permeability with the filler was explained by a competition between the kinetic (diffusivity) and thermodynamic (solubility) contributions defining the permeability process.

 Please wait while we load your content...
Please wait while we load your content...