Novel anion conductors – conductivity, thermodynamic stability and hydration of anion-substituted mayenite-type cage compounds C12A7:X (X = O, OH, Cl, F, CN, S, N)
Abstract
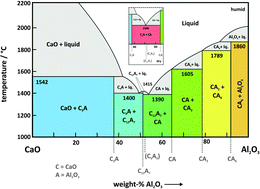
Mayenite (Ca12Al14O33) is a highly interesting functional material not only in view of its unique crystal structure as a cage compound but also for its variety of possible applications. Its ability to incorporate foreign ions into the cage structure opens the possibility to create new types of solid electrolytes and even electrides. Therefore, the conductivity of various anion substituted mayenites was measured as a function of temperature. Due to controversial reports on the stability of mayenite under specific thermodynamic conditions (dry, wet, reducing, and high temperature), a comprehensive study on the stability was performed. Mayenite is clearly not stable under dry conditions (ppm H2O < 100) at temperatures above 1050 °C, and thus, the mayenite phase vanishes from the calcium aluminate phase diagram below a minimum humidity. Two decomposition reactions were observed and are described in detail. To get further insight into the mechanism of hydration of mayenite, the conductivity was measured as a function of water vapour pressure in a range of −5 ≤ lg[pH2O/bar] ≤ −1.6 at temperatures ranging from 1000 °C ≤ θ ≤ 1200 °C. The hydration isotherms are described with high accuracy by the underlying point defect model, which is confirmed in a wide range of water vapour pressure.

 Please wait while we load your content...
Please wait while we load your content...