Electrochemical preparation of nanostructured lanthanum using lanthanum chloride as a precursor in 1-butyl-3-methylimidazolium dicyanamide ionic liquid
Abstract
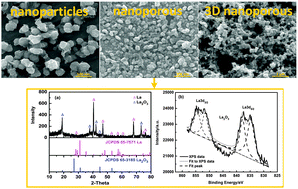
Nanostructured lanthanum was electrochemically prepared on a platinum (Pt) substrate in the room temperature ionic liquid 1-butyl-3-methylimidazolium dicyanamide (BMI-DCA) containing anhydrous LaCl3 at 333 K. The electrochemical reduction behavior of La(III) was investigated using cyclic voltammetry and chronoamperometry techniques. Cyclic voltammogram revealed that the reduction of La(III) in BMI-DCA involved an irreversible process controlled by diffusion. Chronoamperometric transient analysis confirmed the diffusion controlled electrodeposition process with the diffusion coefficient of La(III) species in the range of 10−10 cm2 s−1. The strong complexing capability of DCA− anions facilitated the displacement of chloride ligands and induced the solubility of LaCl3. The subsequent coordination of La(III) and DCA− anions forming [La(DCA)4]− complex anions was monitored by designing amperometric titration experiments. Potentiostatically deposited La-deposits with different nanostructures were characterized by SEM, XRD and XPS analyses. The electrodeposition potential was found to play an important role in controlling the nucleation and growth kinetics of the nanocrystal during the electrodeposition process. Depending on the deposition potential, metallic lanthanum with either nanoparticles or nanoporous structures was obtained.

 Please wait while we load your content...
Please wait while we load your content...