Large protonation-gated photochromism of an OPE-embedded difurylperfluorocyclopentene†
Abstract
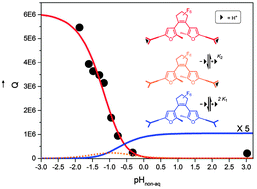
A recently reported protolytic gating effect on the ring closing reaction of an oligo(phenylene ethynylene) (OPE) embedded difurylperfluorocyclopentene (S) with a dimethylaminophenyl chain link in each of the side arms, was quantitatively analyzed in detail. The reaction system (So, SoH+, SoH22+, Sc, ScH+, ScH22+) comprising three protolytic forms in both open and closed configuration, is characterized by four protolytic equilibrium constants and six photochemical quantum yields of ring closing and ring opening. The absorption spectra, conductivity, and effective photochemical quantum yields were measured in acetonitrile as functions of solvent acidity varied by addition of trifluoroacetic acid and triethylamine and characterized by an effective pHnon-aq. Based on the derivation of a rigorous method for assessing the individual quantum yields of ring closure and ring opening of the six species, it was shown that it is specifically the second protonation step that is responsible for a more than 10-fold increase in the quantum yield of ring closure.

 Please wait while we load your content...
Please wait while we load your content...