Relationships between dipole moments of diatomic molecules†
Abstract
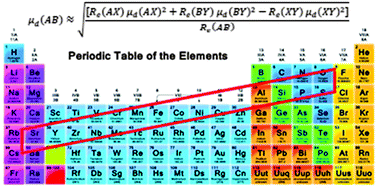
The dipole moment is one of the most important physical properties of a molecule. We present a combination rule for the dipole moments of related diatomic molecules. For molecules AB, AX, BY, and XY from two different element groups in the periodic table, if their elements make a small parallelogram, reliable predictions can be obtained. Our approach is particularly useful for systems with heavy atoms. For a large set of molecules tested, the average difference of the prediction from experimental data is less than 0.2 debye (D). The dipole moments for heavy molecules such as GaCl, InBr, SrCl, and SrS, for which no experimental data are available at present, are predicted to be 3.17, 3.76, 3.85 and 11.54 D, respectively.

 Please wait while we load your content...
Please wait while we load your content...