Nucleation and growth kinetics of zirconium-oxo-alkoxy nanoparticles
Abstract
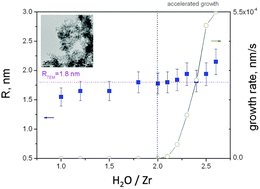
Nucleation and growth of zirconium-oxo-alkoxy (ZOA) nanoparticles were studied in a sol–gel process in n-propanol solution at a hydrolysis ratio H between 1.0 and 2.7 and zirconium-n-propoxyde precursor concentrations between 0.10 and 0.15 mol l−1. The chemical transformations were conducted in quasi-perfect micromixing conditions (Damköhler number Da ≤ 1) and the nanoparticle size evolution was monitored in situ with the light scattering method. The size of primary nanoparticles (nuclei) 2R0 = 3.6 nm was found to be almost independent of the preparation conditions. A remarkable similarity with the titanium-oxo-alkoxy (TOA) nanoparticles was observed. In particular, both systems show the induction stage of the sol–gel growth for a hydrolysis ratio H > 2.0 and stable oxometallate units for H ≤ 2.0. However in contrast to TOA, no stable hierarchical ZOA units (clusters) with R0 ≥ R ≥ 1.0 nm were observed, which makes this system less stable against aggregation, leading to polydispersed nanoparticles.

 Please wait while we load your content...
Please wait while we load your content...