Exploring the binding mechanisms of MIF to CXCR2 using theoretical approaches†
Abstract
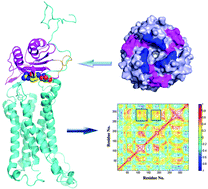
Macrophage migration inhibitory factor (MIF) is a multi-functional protein that acts as a cytokine and as an enzyme. Recently, MIF was identified as a non-canonical ligand of G protein-coupled chemokine receptor CXCR2 with low nanomolar affinity in leukocyte arrest and chemotaxis, but the precise knowledge of the molecular determinants of the MIF–CXCR2 interface has remained unknown. Therefore, we employed homology modeling, protein–protein docking, molecular dynamics (MD) simulations, Molecular Mechanics/Generalized Born Surface Area (MM/GBSA) binding free energy calculations and MM/GBSA binding free energy decomposition to obtain insights into the molecular recognition of MIF with CXCR2. The predicted binding pattern of MIF–CXCR2 is in good agreement with the experimental data and sheds light on the functional role of important MIF–CXCR2 interface residues in association with binding and signaling. According to our predictions, the R11A/D44A double mutations of MIF exhibit a pronounced defect in the binding affinity of MIF to CXCR2, resulting in large conformational changes. The potential two-site binding model for the MIF–CXCR2 recognition was proposed: initialized primarily by the non-polar interactions including the van der Waals and hydrophobic interactions, the N-terminal region of CXCR2 contacts the N-like loop and β-sheet of MIF (site 1), and then the ECL2 and ECL3 regions of CXCR2 form strong interactions with the pseudo-(E)LR motif and C-terminus of MIF, which induces the molecular thermodynamic motion of TMs for signal transduction (site 2). This study will extend our understanding to the binding mechanisms of MIF to CXCR2 and provide useful information for the rational design of potent inhibitors selectively targeting the MIF–CXCR2 interactions.

 Please wait while we load your content...
Please wait while we load your content...