Preparation of a silver nanoparticle-based dual-functional sensor using a complexation–reduction method†
Abstract
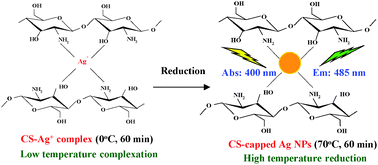
A dual-functional sensor based on silver nanoparticles was synthesized by a two-stage procedure consisting of a low-temperature chitosan–Ag+ complexation followed by a high-temperature reduction of the complex to form chitosan-capped silver nanoparticles (CS-capped Ag NPs). The surface plasmon resonance (SPR) absorption and fluorescence emission of the silver nanoparticles were influenced by the concentration and degradation time of chitosan, and the temperatures of the complexation and reduction reactions. The SPR absorption band was blue-shifted while the intensities of emission and absorption were decreased after reacting the silver nanoparticles with Hg2+ ions. The silver nanoparticles reacted with Hg2+ were characterized by high resolution transmission electron microscopy (HRTEM), energy dispersive spectroscopy (EDS), X-ray photoelectron spectroscopy (XPS), and surface-enhanced Raman scattering spectroscopy (SERS). The results suggested that the particle growth and aggregation of the silver nanoparticles were caused by the adsorption of Hg2+ and deposition of Hg0 on the nanoparticle surface. Direct correlations of the SPR absorption and fluorescence emission with the concentration of Hg2+ were useful for quantitative analysis of Hg2+. It was possible to use the dual-functional silver nanoparticles as a colorimetric and fluorescent sensor for sensitive and selective detection of Hg2+ ions.
- This article is part of the themed collection: Surface-enhanced spectroscopies

 Please wait while we load your content...
Please wait while we load your content...