Site and chirality selective chemical modifications of boron nitride nanotubes (BNNTs) via Lewis acid–base interactions†
Abstract
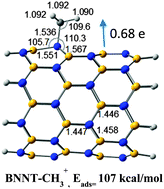
The pristine BNNTs contain both Lewis acid (boron) and Lewis base (nitrogen) centers at their surface. Interactions of ammonia and borane molecules, representatives of Lewis base and acid as adsorbates respectively, with matching sites at the surface of BNNTs, have been explored in the present DFT study. Adsorption energies suggest stronger chemisorption (about 15–20 kcal mol−1) of borane than ammonia (about 5–10 kcal mol−1) in both armchair (4,4) and zigzag (8,0) variants of the tube. NH3 favors (8,0) over the (4,4) tube, whereas BH3 exhibits the opposite preference, indicating some chirality dependence on acid–base interactions. A new feature of bonding is found in BH3/AlH3–BNNTs (at the edge site) complexes, where one hydrogen of the guest molecule is involved in three-center two-electron bonding, in addition to dative covalent bond (N: → B). This interaction causes a reversal of electron flow from borane/alane to BNNT, making the tube an electron acceptor, suggesting tailoring of electronic properties could be possible by varying strength of incoming Lewis acids. On the contrary, BNNTs always behave as electron acceptor in ammonia complexes. IR, XPS and NMR spectra show some characteristic features of complexes and can help experimentalists to identify not only structures of such complexes but also the location of the guest molecules and design second functionalizations. Interaction with several other neutral BF3, BCl3, BH2CH3 and ionic CH3+ acids as well as amino group (CH3NH2 and NH2COOH) were also studied. The strongest interaction (>100 kcal mol−1) is found in BNNT–CH3+ complexes and H-bonds are the only source of stability of NH2COOH–BNNT complexes.

 Please wait while we load your content...
Please wait while we load your content...