The origin of the absorption spectra of porphyrin N- and dithiaporphyrin S-oxides in their neutral and protonated states†
Abstract
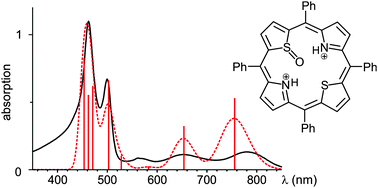
meso-Tetraphenylporphyrin N-oxide (1) and meso-tetraphenyl-21,23-dithiaporphyrin S-oxide (3) possess optical spectra that are distinctly different from their parent porphyrins, meso-tetraphenylporphyrin (2) and meso-tetraphenyl-21,23-dithiaporphyrin (4), respectively. The hyperporphyrin spectra were reproduced and classified using TD CAM-B3LYP and SCS-CC2 computational methods. Calculations revealed the electronic and conformational influences of the N- and S-oxide functionalities. While the N-oxide under acidic conditions forms a dication with a UV-vis spectrum that is nearly indistinguishable from that of the diprotonated parent porphyrin, the diprotonated S-oxide possesses a much different UV-vis spectrum from diprotonated parent dithiaporphyrin. A computational study of the protonation events revealed the site and degree of protonation and rationalized the regular and hyperporphyrin UV-vis spectra of the neutral and protonated species, respectively. The study illuminates the electronic effects of the relatively rare modification of the inner porphyrin heteroatoms. It also illustrates a case in which TD CAM-B3LYP reaches its limits to make reliable predictions about the optical properties of a porphyrinoid, making the use of higher methods essential.

 Please wait while we load your content...
Please wait while we load your content...