Phenolic resin-grafted reduced graphene oxide as a highly stable anode material for lithium ion batteries
Abstract
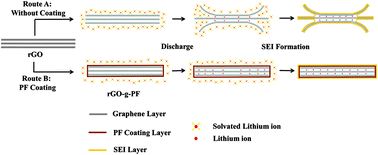
A novel and effective route for preparing phenol formaldehyde resin grafted reduced graphene oxide (rGO-g-PF) electrode materials with highly enhanced electrochemical properties is reported. In order to prepare rGO-g-PF, hydroxymethyl-terminated PF is initially grafted to graphene oxide (GO) via esterification reaction. Subsequently, the grafted GO is reduced by the carbonization process under an inert gas atmosphere. The covalent linkage, morphology, thermal stability and electrochemical properties of rGO-g-PF are systematically investigated by Fourier transform infrared spectroscopy, scanning electron microscopy, thermal gravimetric analysis, differential scanning calorimetry and a variety of electrochemical testing techniques. In the constructed architecture, the amorphous carbon shell can inhibit the co-intercalation of solvated lithium ion and avoid partial exfoliation of the graphene layers, thus effectively reducing the irreversible capacity and preserving the structural integrity. Meanwhile, the carbon coating layer leading to a decreased thickness of SEI film can improve the conductivity of electrode materials. As a result, the rGO-g-PF electrode exhibits impressive high cycling stability at various large current densities (376.5 mA h g−1 at 50 mA g−1 for 250 cycles, 337.8 mA h g−1 at 200 mA g−1 and 267.8 mA h g−1 at 1 A g−1 for 200 cycles), in combination with high rate capability.

 Please wait while we load your content...
Please wait while we load your content...