Influence of zeolite pore structure on product selectivities for protolysis and hydride transfer reactions in the cracking of n-pentane
Abstract
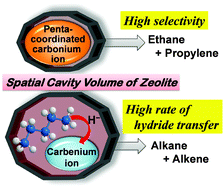
The conversion of n-pentane was carried out to examine the effects of reaction conditions on changes in product selectivities at 823 K, using zeolites with 10- and 12-membered rings. We also investigated the influence of the pore structure of these zeolites on their catalytic activities for both protolysis and hydride transfer reactions. In the first half of this work, we examined the influence of acidic proton concentration and n-pentane pressure on the reaction rates for protolysis and hydride transfer reactions using ZSM-5 zeolites. The rates of hydride transfer reactions were more influenced by pentane pressure compared to protolysis reactions, and were proportional to the square of n-pentane pressure and the concentration of acidic protons. In the second half of this work, the influence of the zeolite pore structure on changes in product selectivities with n-pentane conversion and that on the rates of protolysis and the hydride transfer reactions were revealed using various zeolites with 10- and 12-membered rings. The catalytic activities of zeolites for the protolysis and hydride transfer reactions were influenced more by the spatial volume of the zeolite cavity than the acid strength of protons on the zeolite.

 Please wait while we load your content...
Please wait while we load your content...