The vibrational relaxation of NO in Ar: tunneling in a curve-crossing mechanism
Abstract
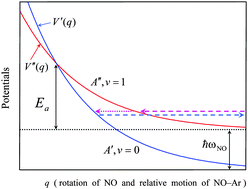
Experimental data for the vibrational relaxation NO(X2Π, v = 1) + Ar → NO(X2Π, v = 0) + Ar between 300 and 2000 K are analyzed. The measured rate coefficients k10 greatly exceed Landau–Teller values LTk10. This observation can be attributed to a mechanism involving curve-crossing of the (A′′, v = 1) and (A′, v = 0) vibronic states of the collision system. At high temperatures, the rate coefficients k10 are well represented by the thermally averaged Landau–Zener rate constant LZk10 with an apparent Arrhenius activation energy Ea/kB near 4500 K. At intermediate temperatures, around T = 900 K, the measured k10 values are a factor of two higher than the extrapolated LZk10 values. This deviation is attributed to tunneling in nonadiabatic curve-crossing transitions, which are analyzed within the Airy approximation (linear model for crossing diabatic curves) and an effective mass approach. This suggests a substantial contribution of hindered rotation of NO to the nonadiabatic perturbation. The extrapolation of the Airy probabilities to even lower temperatures (by the Landau–Lifshitz WKB tunneling expression for simple nonlinear model potentials) indicates a further marked increase of the tunneling contribution beyond the extrapolated LZk10. Near 300 K, the k10 can be two to three orders of magnitude higher than the extrapolated LZk10. This agrees with the limited available experimental data for NO–Ar relaxation near room temperature.

 Please wait while we load your content...
Please wait while we load your content...