Molecular understanding of ion specificity at the peptide bond†
Abstract
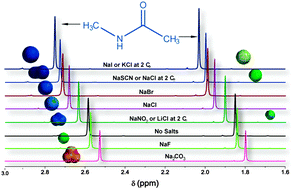
The Hofmeister series has remained a mystery for more than a century. A detailed understanding of the interactions in ion-dissolved systems is still needed because the classical theories have failed to accommodate the specific ion effects. In this study, the interactions between ions, solvent and a model compound for proteins were explored using a direct nuclear magnetic resonance (NMR) approach along with density functional theory (DFT) calculations. It was found that the chaotropic anions caused increasing chemical shifts of the model compound, while kosmotropic anions resulted in decreasing shifts; this suggests that the kosmotropic anions were prevented from interacting with the model compound. The experimental results can be explained by a combination of local electrostatic interactions and hydrogen bonding. Although more effort are required to justify the NMR method applied in this study, the results could give a quantitative standard for defining kosmotropes/chaotropes and might provide a new way for predicting the effects of unfamiliar ions in the future.

 Please wait while we load your content...
Please wait while we load your content...