Aggregation-induced chiral symmetry breaking of a naphthalimide–cyanostilbene dyad†
Abstract
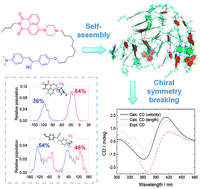
Spontaneously emerged supramolecular chirality and chiral symmetry breaking from achiral/racemic constituents remain poorly understood. We here report that supramolecular chirality may emerge from the structural flexibility of achiral aryl nitrogen centres which provide instantaneous chirality. Employing a naphthalimide–cyanostilbene dyad as a model, we explored the underlying mechanism of aggregation-induced chiral symmetry breaking and found that the conformations of the N-naphthylpiperazine and the N,N-dimethylaniline units facilitate the formation of ordered supramolecular structures and offer opposite handedness. Furthermore, chiral symmetry breaking of the monomers was amplified by the formation of dimers. The microscopic and the macroscopic observations from the theoretical simulations and experimental measurements are thus rationalized by connecting the population of the dihedral angles of the aryl nitrogen centres, the morphology of the self-assemblies, and the observed circular dichroism spectra.

 Please wait while we load your content...
Please wait while we load your content...