Resolving the anomalous infrared spectrum of the MeCN–HCl molecular cluster using ab Initio molecular dynamics†
Abstract
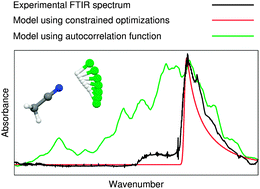
We present a molecular dynamics (MD) based study of the acetonitrile–hydrogen chloride molecular cluster in the gas phase, aimed at resolving the anomalous features often seen in infrared spectra of hydrogen bonded complexes. We find that the infrared spectrum obtained from the Fourier transform of the electric dipole moment autocorrelation function converges very slowly due to the floppy nature of the complex. Even after 55 picoseconds of simulation, significant differences in the modelled and experimental spectrum are seen, likely due to insufficient configurational sampling. Instead, we utilize the MD trajectory for a structural based analysis. We find that the most populated values of the N–H–Cl angle are around 162°. The global minimum energy conformation at 180.0° is essentially unpopulated. We re-model the spectrum by combining population data from the MD simulations with optimizations constraining the N–H–Cl angle. This re-modelled spectrum is in excellent accordance with the experimental spectrum and we conclude that the observed spectral anomaly is due to the dynamics of the N–H–Cl angle.

 Please wait while we load your content...
Please wait while we load your content...