Assessment of density-functionals for describing the X− + CH3ONO2 gas-phase reactions with X = F, OH, CH2CN†
Abstract
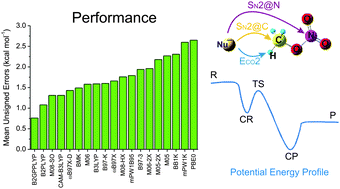
The energetics of the ECO2, SN2@C and SN2@N channels of X− + CH3ONO2 (X = F, OH, CH2CN) gas-phase reactions were computed using the CCSD(T)/CBS method. This benchmark extends a previous study with X = OH [M. A. F. de Souza et al., J. Am. Chem. Soc., 2012, 134, 19004] and was used to ascertain the accuracy and robustness of nineteen density-functionals for describing these potential energy profiles (PEP) as well as the kinetic product distributions obtained from RRKM calculations. Assessments were based on the mean unsigned error (MUE), the mean signed error (MSE), the #best : #worst (BW) criterion and the statistical confidence interval (CI) for the MSE. In general, double-hybrid (DH) functionals perform better than the range-separated ones, and both are better than the global-hybrid functionals. Based on the MUE and CI criteria the B2GPPLYP, B2PLYP, M08-SO, BMK, ωB97X-D, CAM-B3LYP, M06, M08-HX, ωB97X and B97-K functionals show the best performance in the description of these PEPs. Within this set, the B2GPPLYP functional is the most accurate and robust. The RRKM results indicate that the DHs are the best for describing the selectivities of these reactions. Compared to CCSD(T), the B2PLYP method has a relative error of only ca. 1% for the selectivity and the accuracy to provide the correct conclusion concerning the nonstatistical behavior of these reactions.

 Please wait while we load your content...
Please wait while we load your content...