Mesoscopic modeling of Li insertion in phase-separating electrode materials: application to lithium iron phosphate
Abstract
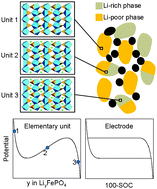
A simple mesoscopic model is presented which accounts for the inhomogeneity of physical properties and bi-stable nature of phase-change insertion materials used in battery electrodes. The model does not include any geometric detail of the active material and discretizes the total active material domain into meso-scale units featuring basic thermodynamic (non-monotonic equilibrium potential as a function of Li content) and kinetic (insertion–de-insertion resistance) properties. With only these two factors incorporated, the model is able to simultaneously capture unique phenomena including the memory effect observed in lithium iron phosphate electrodes. The analysis offers a new physical insight into modeling of phase-change active materials which are of special interest for use in high power Li-ion batteries.

 Please wait while we load your content...
Please wait while we load your content...