Gibbs free energy of liquid water derived from infrared measurements†
Abstract
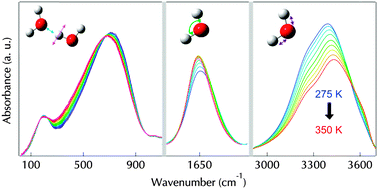
Infrared spectra of pure liquid water were recorded from 20 cm−1 to 4000 cm−1 at temperatures ranging from 263 K to 363 K. The evolution of connectivity, libration, bending and OH stretching bands as a function of temperature follows the evolution of the inter-molecular dynamics, and so gives insight into the internal energy averaged over the measurement time and space. A partition function, which takes into account the inter-molecular and intra-molecular modes of vibration of water, all variable with the molecular networking, was developed to convert this vibrational absorption behavior of water into its macroscopic Gibbs free energy, assuming the vibrational energy to feature most of the water energy. Calculated Gibbs free energies along the thermal range are in close agreement with the literature values up to 318 K. Above this temperature, contributions specific to the non H-bonded molecules must be involved to closely fit the thermodynamics of water. We discussed this temperature threshold in relation to the well-known isosbestic point. Generally speaking, our approach is valuable to convert the IR molecular data into mean field properties, a quantitative basis to predict how water behaves in natural or industrial settings.

 Please wait while we load your content...
Please wait while we load your content...